To Go Directly to the Table of Neoplasms
| Tumour | |
|---|---|
| Other names | Tumor, neoplasm, carcinocytes |
 | |
| Colectomy specimen containing a malignant neoplasm, namely an invasive example of colorectal cancer (the crater-similar, reddish, irregularly shaped tumor) | |
| Specialty | Oncology |
| Symptoms | Lump |
| Complications | Cancer |
| Causes | Radiations, environmental factor, certain infections |
A neoplasm ([1]) is a type of abnormal and excessive growth of tissue. The procedure that occurs to course or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists in growing abnormally, even if the original trigger is removed.[2] [3] [4] This aberrant growth usually forms a mass, when it may exist called a tumor.[five]
ICD-10 classifies neoplasms into 4 principal groups: benign neoplasms, in situ neoplasms, malignant neoplasms, and neoplasms of uncertain or unknown behavior.[6] Malignant neoplasms are likewise simply known every bit cancers and are the focus of oncology.
Prior to the abnormal growth of tissue, as neoplasia, cells often undergo an abnormal design of growth, such equally metaplasia or dysplasia.[7] However, metaplasia or dysplasia does not always progress to neoplasia and can occur in other conditions as well.[2] The word is from Ancient Greek νέος- neo 'new' and πλάσμα plasma 'formation, creation'.
Types [edit]
A neoplasm tin be benign, potentially malignant, or cancerous (cancer).[eight]
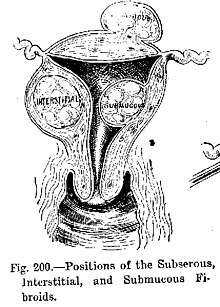
- Benign tumors include uterine fibroids, osteophytes and melanocytic nevi (skin moles). They are circumscribed and localized and exercise not transform into cancer.[vii]
- Potentially-malignant neoplasms include carcinoma in situ. They are localised, do not invade and destroy but in time, may transform into a cancer.
- Cancerous neoplasms are commonly called cancer. They invade and destroy the surrounding tissue, may form metastases and, if untreated or unresponsive to handling, will generally prove fatal.
- Secondary neoplasm refers to any of a class of malignant tumor that is either a metastatic offshoot of a chief tumor, or an apparently unrelated tumor that increases in frequency following certain cancer treatments such as chemotherapy or radiotherapy.
- Rarely there can be a metastatic tumour with no known site of the chief cancer and this is classed as a cancer of unknown primary origin.
Clonality [edit]
Neoplastic tumors are often heterogeneous and contain more than one blazon of cell, but their initiation and connected growth is usually dependent on a unmarried population of neoplastic cells. These cells are presumed to exist clonal – that is, they are derived from the same cell,[9] and all carry the aforementioned genetic or epigenetic bibelot – evident of clonality. For lymphoid neoplasms, e.m. lymphoma and leukemia, clonality is proven by the distension of a single rearrangement of their immunoglobulin gene (for B prison cell lesions) or T cell receptor gene (for T cell lesions). The demonstration of clonality is now considered to be necessary to identify a lymphoid cell proliferation as neoplastic.[10]
Information technology is tempting to ascertain neoplasms every bit clonal cellular proliferations but the sit-in of clonality is not e'er possible. Therefore, clonality is not required in the definition of neoplasia.[ citation needed ]
Neoplasm vs. tumor [edit]
The word tumor or tumour comes from the Latin discussion for swelling, which is one of the cardinal signs of inflammation. The give-and-take originally referred to whatsoever form of swelling, neoplastic or not. In modernistic English, tumor is used as a synonym for neoplasm (a solid or fluid-filled cystic lesion that may or may non exist formed by an aberrant growth of neoplastic cells) that appears enlarged in size.[11] [12] Some neoplasms do not form a tumor - these include leukemia and most forms of carcinoma in situ. Tumor is also not synonymous with cancer. While cancer is by definition cancerous, a tumor can exist benign, precancerous, or cancerous.
The terms mass and nodule are ofttimes used synonymously with tumor. Generally speaking, however, the term tumor is used generically, without reference to the physical size of the lesion.[ii] More specifically, the term mass is ofttimes used when the lesion has a maximal diameter of at least 20 millimeters (mm) in greatest direction, while the term nodule is usually used when the size of the lesion is less than 20 mm in its greatest dimension (25.four mm = ane inch).[2]
Causes [edit]
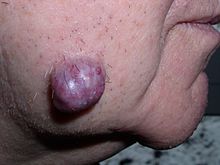
Tumors in humans occur equally a outcome of accumulated genetic and epigenetic alterations within single cells, which cause the prison cell to dissever and aggrandize uncontrollably.[13] A neoplasm tin can be caused past an abnormal proliferation of tissues, which can be caused by genetic mutations. Non all types of neoplasms cause a tumorous overgrowth of tissue, nevertheless (such every bit leukemia or carcinoma in situ) and similarities between neoplasmic growths and regenerative processes, east.g., dedifferentiation and rapid jail cell proliferation, take been pointed out.[14]
Tumor growth has been studied using mathematics and continuum mechanics. Vascular tumors such as hemangiomas and lymphangiomas (formed from blood or lymph vessels) are thus looked at as existence amalgams of a solid skeleton formed by pasty cells and an organic liquid filling the spaces in which cells tin grow.[15] Nether this type of model, mechanical stresses and strains tin can be dealt with and their influence on the growth of the tumor and the surrounding tissue and vasculature elucidated. Recent findings from experiments that use this model prove that active growth of the tumor is restricted to the outer edges of the tumor and that stiffening of the underlying normal tissue inhibits tumor growth too.[xvi]
Benign atmospheric condition that are not associated with an abnormal proliferation of tissue (such as sebaceous cysts) can as well present as tumors, however, only have no malignant potential. Breast cysts (as occur normally during pregnancy and at other times) are some other case, as are other encapsulated glandular swellings (thyroid, adrenal gland, pancreas).
Encapsulated hematomas, encapsulated necrotic tissue (from an insect bite, foreign torso, or other baneful mechanism), keloids (discrete overgrowths of scar tissue) and granulomas may besides nowadays equally tumors.
Detached localized enlargements of normal structures (ureters, claret vessels, intrahepatic or extrahepatic biliary ducts, pulmonary inclusions, or gastrointestinal duplications) due to outflow obstructions or narrowings, or aberrant connections, may besides present as a tumor. Examples are arteriovenous fistulae or aneurysms (with or without thrombosis), biliary fistulae or aneurysms, sclerosing cholangitis, cysticercosis or hydatid cysts, abdominal duplications, and pulmonary inclusions as seen with cystic fibrosis. It tin can be dangerous to biopsy a number of types of tumor in which the leakage of their contents would potentially be catastrophic. When such types of tumors are encountered, diagnostic modalities such equally ultrasound, CT scans, MRI, angiograms, and nuclear medicine scans are employed prior to (or during) biopsy or surgical exploration/excision in an attempt to avert such severe complications.[ citation needed ]
Cancerous neoplasms [edit]
Deoxyribonucleic acid damage [edit]

The primal role of Deoxyribonucleic acid damage and epigenetic defects in DNA repair genes in malignant neoplasms
DNA impairment is considered to be the main underlying cause of malignant neoplasms known as cancers.[17] Its fundamental office in progression to cancer is illustrated in the figure in this section, in the box virtually the top. (The cardinal features of DNA damage, epigenetic alterations and scarce DNA repair in progression to cancer are shown in red.) DNA damage is very common. Naturally occurring DNA amercement (mostly due to cellular metabolism and the properties of Deoxyribonucleic acid in water at body temperatures) occur at a rate of more than threescore,000 new damages, on average, per man cell, per day[ citation needed ] [also see article DNA damage (naturally occurring) ]. Additional Deoxyribonucleic acid amercement can arise from exposure to exogenous agents. Tobacco smoke causes increased exogenous DNA damage, and these Dna amercement are the likely cause of lung cancer due to smoking.[18] UV lite from solar radiation causes DNA damage that is important in melanoma.[xix] Helicobacter pylori infection produces high levels of reactive oxygen species that damage Deoxyribonucleic acid and contributes to gastric cancer.[20] Bile acids, at high levels in the colons of humans eating a loftier fat diet, besides cause DNA damage and contribute to colon cancer.[21] Katsurano et al. indicated that macrophages and neutrophils in an inflamed colonic epithelium are the source of reactive oxygen species causing the DNA damages that initiate colonic tumorigenesis.[22] [ unreliable source? ] Some sources of Dna impairment are indicated in the boxes at the top of the figure in this section.
Individuals with a germ line mutation causing deficiency in whatever of 34 DNA repair genes (run into commodity DNA repair-deficiency disorder) are at increased take a chance of cancer. Some germ line mutations in Deoxyribonucleic acid repair genes cause up to 100% lifetime chance of cancer (e.1000., p53 mutations).[23] These germ line mutations are indicated in a box at the left of the figure with an pointer indicating their contribution to Dna repair deficiency.
Near lxx% of malignant neoplasms have no hereditary component and are called "sporadic cancers".[24] Only a minority of sporadic cancers have a deficiency in DNA repair due to mutation in a Deoxyribonucleic acid repair gene. However, a majority of desultory cancers have deficiency in DNA repair due to epigenetic alterations that reduce or silence DNA repair gene expression. For case, of 113 sequential colorectal cancers, only four had a missense mutation in the Dna repair gene MGMT, while the majority had reduced MGMT expression due to methylation of the MGMT promoter region (an epigenetic alteration).[25] Five reports present evidence that betwixt 40% and xc% of colorectal cancers have reduced MGMT expression due to methylation of the MGMT promoter region.[26] [27] [28] [29] [thirty]
Similarly, out of 119 cases of mismatch repair-deficient colorectal cancers that lacked Deoxyribonucleic acid repair gene PMS2 expression, PMS2 was deficient in 6 due to mutations in the PMS2 cistron, while in 103 cases PMS2 expression was deficient because its pairing partner MLH1 was repressed due to promoter methylation (PMS2 protein is unstable in the absence of MLH1).[31] In the other ten cases, loss of PMS2 expression was likely due to epigenetic overexpression of the microRNA, miR-155, which down-regulates MLH1.[32]
In further examples, epigenetic defects were found at frequencies of between 13%-100% for the Deoxyribonucleic acid repair genes BRCA1, WRN, FANCB, FANCF, MGMT, MLH1, MSH2, MSH4, ERCC1, XPF, NEIL1 and ATM. These epigenetic defects occurred in various cancers (e.k. chest, ovarian, colorectal and caput and neck). Two or iii deficiencies in expression of ERCC1, XPF or PMS2 occur simultaneously in the majority of the 49 colon cancers evaluated by Facista et al.[33] Epigenetic alterations causing reduced expression of Deoxyribonucleic acid repair genes is shown in a key box at the third level from the top of the figure in this section, and the consequent DNA repair deficiency is shown at the 4th level.
When expression of Deoxyribonucleic acid repair genes is reduced, Deoxyribonucleic acid damages accumulate in cells at a higher than normal level, and these excess damages cause increased frequencies of mutation or epimutation. Mutation rates strongly increase in cells lacking in DNA mismatch repair[34] [35] or in homologous recombinational repair (HRR).[36]
During repair of Dna double strand breaks, or repair of other DNA damages, incompletely cleared sites of repair can crusade epigenetic cistron silencing.[37] [38] Deoxyribonucleic acid repair deficiencies (level 4 in the figure) cause increased DNA amercement (level five in the effigy) which result in increased somatic mutations and epigenetic alterations (level half-dozen in the figure).
Field defects, normal appearing tissue with multiple alterations (and discussed in the section below), are common precursors to development of the disordered and improperly proliferating clone of tissue in a malignant neoplasm. Such field defects (2nd level from bottom of effigy) may have multiple mutations and epigenetic alterations.
Once a cancer is formed, it usually has genome instability. This instability is likely due to reduced Deoxyribonucleic acid repair or excessive Deoxyribonucleic acid damage. Because of such instability, the cancer continues to evolve and to produce sub clones. For example, a renal cancer, sampled in 9 areas, had 40 ubiquitous mutations, demonstrating tumor heterogeneity (i.e. present in all areas of the cancer), 59 mutations shared by some (simply not all areas), and 29 "individual" mutations simply present in ane of the areas of the cancer.[39]
Field defects [edit]
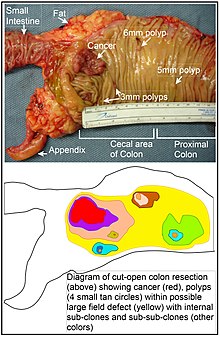
Longitudinally opened freshly resected colon segment showing a cancer and four polyps, plus a schematic diagram indicating a likely field defect (a region of tissue that precedes and predisposes to the development of cancer) in this colon segment. The diagram indicates sub-clones and sub-sub-clones that were precursors to the tumors.
Diverse other terms have been used to draw this phenomenon, including "field consequence", "field cancerization", and "field carcinogenesis". The term "field cancerization" was start used in 1953 to draw an area or "field" of epithelium that has been preconditioned past (at that time) largely unknown processes so as to predispose information technology towards development of cancer.[40] Since then, the terms "field cancerization" and "field defect" have been used to draw pre-malignant tissue in which new cancers are probable to arise.[ citation needed ]
Field defects are important in progression to cancer.[41] [42] However, in most cancer enquiry, equally pointed out by Rubin[43] "The vast majority of studies in cancer research has been done on well-divers tumors in vivo, or on detached neoplastic foci in vitro. However there is evidence that more than eighty% of the somatic mutations institute in mutator phenotype homo colorectal tumors occur before the onset of concluding clonal expansion.[44] Similarly, Vogelstein et al.[45] point out that more than than half of somatic mutations identified in tumors occurred in a pre-neoplastic phase (in a field defect), during growth of apparently normal cells. Likewise, epigenetic alterations present in tumors may have occurred in pre-neoplastic field defects.[ commendation needed ]
An expanded view of field effect has been termed "etiologic field effect", which encompasses non only molecular and pathologic changes in pre-neoplastic cells but also influences of exogenous environmental factors and molecular changes in the local microenvironment on neoplastic development from tumor initiation to patient death.[46]
In the colon, a field defect probably arises past natural selection of a mutant or epigenetically contradistinct prison cell among the stem cells at the base of operations of one of the intestinal crypts on the within surface of the colon. A mutant or epigenetically contradistinct stalk jail cell may replace the other nearby stem cells by natural selection. Thus, a patch of aberrant tissue may arise. The effigy in this department includes a photo of a freshly resected and lengthwise-opened segment of the colon showing a colon cancer and iv polyps. Below the photo, at that place is a schematic diagram of how a large patch of mutant or epigenetically altered cells may accept formed, shown past the big area in yellowish in the diagram. Within this first big patch in the diagram (a big clone of cells), a second such mutation or epigenetic amending may occur so that a given stem cell acquires an advantage compared to other stem cells inside the patch, and this altered stalk cell may expand clonally forming a secondary patch, or sub-clone, within the original patch. This is indicated in the diagram by four smaller patches of different colors within the large yellow original expanse. Inside these new patches (sub-clones), the process may be repeated multiple times, indicated past the nonetheless smaller patches within the 4 secondary patches (with still dissimilar colors in the diagram) which clonally expand, until stalk cells arise that generate either modest polyps or else a malignant neoplasm (cancer).[ citation needed ]
In the photo, an credible field defect in this segment of a colon has generated four polyps (labeled with the size of the polyps, 6mm, 5mm, and two of 3mm, and a cancer well-nigh 3 cm across in its longest dimension). These neoplasms are too indicated, in the diagram below the photo, by 4 minor tan circles (polyps) and a larger cherry area (cancer). The cancer in the photograph occurred in the cecal expanse of the colon, where the colon joins the small intestine (labeled) and where the appendix occurs (labeled). The fatty in the photo is external to the outer wall of the colon. In the segment of colon shown here, the colon was cut open up lengthwise to expose the inner surface of the colon and to display the cancer and polyps occurring within the inner epithelial lining of the colon.[ citation needed ]
If the general process past which sporadic colon cancers arise is the germination of a pre-neoplastic clone that spreads by natural selection, followed by formation of internal sub-clones within the initial clone, and sub-sub-clones inside those, and then colon cancers generally should be associated with, and exist preceded past, fields of increasing abnormality reflecting the succession of premalignant events. The near extensive region of abnormality (the outermost yellow irregular area in the diagram) would reflect the primeval event in germination of a malignant neoplasm.[ citation needed ]
In experimental evaluation of specific DNA repair deficiencies in cancers, many specific DNA repair deficiencies were as well shown to occur in the field defects surrounding those cancers. The Tabular array, below, gives examples for which the DNA repair deficiency in a cancer was shown to be caused by an epigenetic amending, and the somewhat lower frequencies with which the same epigenetically caused Deoxyribonucleic acid repair deficiency was found in the surrounding field defect.
| Cancer | Gene | Frequency in Cancer | Frequency in Field Defect | Ref. |
|---|---|---|---|---|
| Colorectal | MGMT | 46% | 34% | [26] |
| Colorectal | MGMT | 47% | 11% | [28] |
| Colorectal | MGMT | lxx% | threescore% | [47] |
| Colorectal | MSH2 | thirteen% | five% | [28] |
| Colorectal | ERCC1 | 100% | 40% | [33] |
| Colorectal | PMS2 | 88% | 50% | [33] |
| Colorectal | XPF | 55% | 40% | [33] |
| Head and Cervix | MGMT | 54% | 38% | [48] |
| Head and Neck | MLH1 | 33% | 25% | [49] |
| Caput and Neck | MLH1 | 31% | 20% | [50] |
| Stomach | MGMT | 88% | 78% | [51] |
| Stomach | MLH1 | 73% | twenty% | [52] |
| Esophagus | MLH1 | 77%-100% | 23%-79% | [53] |
Some of the modest polyps in the field defect shown in the photo of the opened colon segment may exist relatively benign neoplasms. Of polyps less than 10mm in size, plant during colonoscopy and followed with echo colonoscopies for 3 years, 25% were unchanged in size, 35% regressed or shrank in size while 40% grew in size.[54]
Genome instability [edit]
Cancers are known to exhibit genome instability or a mutator phenotype.[55] The protein-coding Deoxyribonucleic acid within the nucleus is about one.5% of the total genomic DNA.[56] Within this protein-coding Deoxyribonucleic acid (chosen the exome), an average cancer of the chest or colon can accept virtually 60 to 70 protein altering mutations, of which nigh iii or 4 may exist "driver" mutations, and the remaining ones may be "passenger" mutations[45] Yet, the average number of DNA sequence mutations in the entire genome (including non-protein-coding regions) within a chest cancer tissue sample is virtually 20,000.[57] In an average melanoma tissue sample (where melanomas have a higher exome mutation frequency[45]) the total number of Dna sequence mutations is about eighty,000.[58] This compares to the very low mutation frequency of about 70 new mutations in the entire genome betwixt generations (parent to child) in humans.[59] [threescore]
The high frequencies of mutations in the total nucleotide sequences within cancers suggest that oft an early alteration in the field defects giving rise to a cancer (due east.one thousand. yellowish area in the diagram in this section) is a deficiency in Dna repair. The big field defects surrounding colon cancers (extending to at most x cm on each side of a cancer) were shown past Facista et al.[33] to frequently take epigenetic defects in ii or 3 DNA repair proteins (ERCC1, XPF or PMS2) in the unabridged expanse of the field defect. Deficiencies in DNA repair cause increased mutation rates.[34] [35] [36] A deficiency in DNA repair, itself, tin permit DNA damages to accumulate, and mistake-decumbent translesion synthesis past some of those amercement may give rise to mutations. In addition, faulty repair of these accumulated Dna amercement may give ascension to epimutations. These new mutations or epimutations may provide a proliferative advantage, generating a field defect. Although the mutations/epimutations in Deoxyribonucleic acid repair genes do not, themselves, confer a selective advantage, they may be carried along as passengers in cells when the cells acquire additional mutations/epimutations that do provide a proliferative advantage.
Etymology [edit]
The term tumour is a synonym of tumor. Neoplasia denotes the process of the formation of neoplasms/tumors, and the process is referred to equally a neoplastic process. The discussion neoplastic itself comes from Greek neo 'new' and plastic 'formed, molded'.[ citation needed ]
The term tumor derives from the Latin noun tumor 'a swelling', ultimately from the verb tumēre 'to great'. In the British Commonwealth, the spelling tumour is commonly used, whereas in the U.S. the word is usually spelled tumor.[ citation needed ]
In its medical sense, tumor has traditionally meant an abnormal swelling of the flesh. The Roman medical encyclopedist Celsus (c. 30 BC–38 Advertising) described the four central signs of acute inflammation equally tumor, dolor, calor, and rubor (swelling, pain, increased oestrus, and redness). (His treatise, De Medicina, was the first medical book printed in 1478 following the invention of the movable-type printing press.)
In contemporary English, the discussion tumor is oftentimes used as a synonym for a cystic (liquid-filled) growth or solid neoplasm (cancerous or non-cancerous),[61] with other forms of swelling often referred to as "swellings".[62]
Related terms occur ordinarily in the medical literature, where the nouns tumefaction and tumescence (derived from the adjective tumescent),[63] are electric current medical terms for not-neoplastic swelling. This type of swelling is most ofttimes caused by inflammation acquired by trauma, infection, and other factors.
Tumors may be caused by weather condition other than an overgrowth of neoplastic cells, however. Cysts (such as sebaceous cysts) are also referred to as tumors, fifty-fifty though they accept no neoplastic cells. This is standard in medical-billing terminology (especially when billing for a growth whose pathology has nonetheless to exist determined).
See also [edit]
- Somatic evolution in cancer
- List of biological evolution disorders
- Epidemiology of cancer
- Pleomorphism
References [edit]
- ^ "Neoplasm". Lexico. Oxford University Press.
- ^ a b c d Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O (July 2014). "Type-2 pericytes participate in normal and tumoral angiogenesis". Am. J. Physiol., Cell Physiol. 307 (1): C25–38. doi:x.1152/ajpcell.00084.2014. PMC4080181. PMID 24788248.
- ^ Cooper GM (1992). Elements of homo cancer. Boston: Jones and Bartlett Publishers. p. sixteen. ISBN978-0-86720-191-8.
- ^ Taylor, Elizabeth J. (2000). Dorland'south Illustrated medical lexicon (29th ed.). Philadelphia: Saunders. p. 1184. ISBN978-0721662541.
- ^ Stedman's medical dictionary (28th ed.). Philadelphia: Lippincott Williams & Wilkins. 2006. p. Neoplasm. ISBN978-0781733908.
- ^ "Ii Neoplasms". International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) Version for 2010. World Health Arrangement. Retrieved xix June 2014.
- ^ a b Abrams, Gerald. "Neoplasia I". Retrieved 23 Jan 2012.
- ^ "Cancer - Activeness ane - Glossary, page 4 of five". Archived from the original on 2008-05-09. Retrieved 2008-01-08 .
- ^ "Medical Definition of Clone".
- ^ Lee ES, Locker J, Nalesnik M, Reyes J, Jaffe R, Alashari M, Nour B, Tzakis A, Dickman PS (Jan 1995). "The clan of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation". N. Engl. J. Med. 332 (1): 19–25. doi:10.1056/NEJM199501053320104. PMID 7990861.
- ^ "Pancreas Cancer: Glossary of Terms". Retrieved 2008-01-08 .
- ^ "Tumor". Dorland'southward Illustrated Medical Dictionary (31st ed.). Saunders. 2007. ISBN978-1-84972-348-0.
- ^ Tammela, Tuomas; Sage, Julien (2020). "Investigating Tumor Heterogeneity in Mouse Models". Annual Review of Cancer Biological science. 4 (i): 99–119. doi:10.1146/annurev-cancerbio-030419-033413. PMC8218894. PMID 34164589.
- ^ Asashima M, Oinuma T, Meyer-Rochow VB (1987). "Tumors in amphibia". Zoological Science. four: 411–425.
- ^ Ambrosi D, Mollica F (2002). "On the mechanics of a growing tumor". International Periodical of Engineering. xl (12): 1297–316. doi:10.1016/S0020-7225(02)00014-9.
- ^ Volokh KY (September 2006). "Stresses in growing soft tissues". Acta Biomater. 2 (v): 493–504. doi:10.1016/j.actbio.2006.04.002. PMID 16793355.
- ^ Kastan MB (2008). "Deoxyribonucleic acid damage responses: mechanisms and roles in human disease: 2007 G.H.A. Clowes Memorial Laurels Lecture". Mol. Cancer Res. 6 (4): 517–24. doi:ten.1158/1541-7786.MCR-08-0020. PMID 18403632.
- ^ Cunningham FH, Fiebelkorn S, Johnson M, Meredith C (November 2011). "A novel application of the Margin of Exposure approach: segregation of tobacco smoke toxicants". Food Chem. Toxicol. 49 (eleven): 2921–33. doi:10.1016/j.fct.2011.07.019. PMID 21802474.
- ^ Kanavy HE, Gerstenblith MR (December 2011). "Ultraviolet radiation and melanoma". Semin Cutan Med Surg. 30 (four): 222–8. doi:x.1016/j.sder.2011.08.003. PMID 22123420.
- ^ Handa O, Naito Y, Yoshikawa T (2011). "Redox biology and gastric carcinogenesis: the role of Helicobacter pylori". Redox Rep. 16 (ane): ane–vii. doi:x.1179/174329211X12968219310756. PMC6837368. PMID 21605492.
- ^ Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, Bernstein H (August 2011). "Carcinogenicity of deoxycholate, a secondary bile acid". Arch. Toxicol. 85 (8): 863–71. doi:x.1007/s00204-011-0648-vii. PMC3149672. PMID 21267546.
- ^ Katsurano Yard, Niwa T, Yasui Y, Shigematsu Y, Yamashita Due south, Takeshima H, Lee MS, Kim YJ, Tanaka T, Ushijima T (Jan 2012). "Early-stage germination of an epigenetic field defect in a mouse colitis model, and not-essential roles of T- and B-cells in Deoxyribonucleic acid methylation consecration". Oncogene. 31 (three): 342–51. doi:10.1038/onc.2011.241. PMID 21685942.
- ^ Malkin D (Apr 2011). "Li-fraumeni syndrome". Genes Cancer. 2 (4): 475–84. doi:10.1177/1947601911413466. PMC3135649. PMID 21779515.
- ^ Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo Grand, Pukkala E, Skytthe A, Hemminki K (July 2000). "Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Kingdom of denmark, and Finland". N. Engl. J. Med. 343 (two): 78–85. doi:10.1056/NEJM200007133430201. PMID 10891514.
- ^ Halford S, Rowan A, Sawyer E, Talbot I, Tomlinson I (June 2005). "O(6)-methylguanine methyltransferase in colorectal cancers: detection of mutations, loss of expression, and weak association with G:C>A:T transitions". Gut. 54 (6): 797–802. doi:x.1136/gut.2004.059535. PMC1774551. PMID 15888787.
- ^ a b Shen 50, Kondo Y, Rosner GL, Xiao Fifty, Hernandez NS, Vilaythong J, Houlihan PS, Krouse RS, Prasad AR, Einspahr JG, Buckmeier J, Alberts DS, Hamilton SR, Issa JP (September 2005). "MGMT promoter methylation and field defect in sporadic colorectal cancer". J. Natl. Cancer Inst. 97 (18): 1330–8. doi:10.1093/jnci/dji275. PMID 16174854.
- ^ Psofaki V, Kalogera C, Tzambouras N, Stephanou D, Tsianos East, Seferiadis K, Kolios G (July 2010). "Promoter methylation status of hMLH1, MGMT, and CDKN2A/p16 in colorectal adenomas". World J. Gastroenterol. 16 (28): 3553–60. doi:10.3748/wjg.v16.i28.3553. PMC2909555. PMID 20653064.
- ^ a b c Lee KH, Lee JS, Nam JH, Choi C, Lee MC, Park CS, Juhng SW, Lee JH (Oct 2011). "Promoter methylation status of hMLH1, hMSH2, and MGMT genes in colorectal cancer associated with adenoma-carcinoma sequence". Langenbecks Curvation Surg. 396 (7): 1017–26. doi:x.1007/s00423-011-0812-ix. PMID 21706233. S2CID 8069716.
- ^ Amatu A, Sartore-Bianchi A, Moutinho C, Belotti A, Bencardino Yard, Chirico G, Cassingena A, Rusconi F, Esposito A, Nichelatti M, Esteller M, Siena S (Apr 2013). "Promoter CpG island hypermethylation of the DNA repair enzyme MGMT predicts clinical response to dacarbazine in a phase 2 study for metastatic colorectal cancer". Clin. Cancer Res. 19 (8): 2265–72. doi:10.1158/1078-0432.CCR-12-3518. PMID 23422094.
- ^ Mokarram P, Zamani M, Kavousipour Southward, Naghibalhossaini F, Irajie C, Moradi Sarabi M, et al. (May 2013). "Different patterns of Dna methylation of the two distinct O6-methylguanine-DNA methyltransferase (O6-MGMT) promoter regions in colorectal cancer". Mol. Biol. Rep. twoscore (5): 3851–7. doi:10.1007/s11033-012-2465-3. PMID 23271133. S2CID 18733871.
- ^ Truninger 1000, Menigatti M, Luz J, Russell A, Haider R, Gebbers JO, et al. (May 2005). "Immunohistochemical analysis reveals high frequency of PMS2 defects in colorectal cancer". Gastroenterology. 128 (v): 1160–71. doi:ten.1053/j.gastro.2005.01.056. PMID 15887099.
- ^ Valeri Due north, Gasparini P, Fabbri Thousand, Braconi C, Veronese A, Lovat F, et al. (April 2010). "Modulation of mismatch repair and genomic stability past miR-155". Proc. Natl. Acad. Sci. The statesA. 107 (fifteen): 6982–7. Bibcode:2010PNAS..107.6982V. doi:10.1073/pnas.1002472107. PMC2872463. PMID 20351277.
- ^ a b c d e Facista A, Nguyen H, Lewis C, Prasad AR, Ramsey Fifty, Zaitlin B, et al. (2012). "Deficient expression of Deoxyribonucleic acid repair enzymes in early on progression to desultory colon cancer". Genome Integr. three (1): 3. doi:10.1186/2041-9414-3-3. PMC3351028. PMID 22494821.
- ^ a b Narayanan L, Fritzell JA, Baker SM, Liskay RM, Glazer PM (April 1997). "Elevated levels of mutation in multiple tissues of mice deficient in the DNA mismatch repair factor Pms2". Proc. Natl. Acad. Sci. U.S.A. 94 (7): 3122–7. Bibcode:1997PNAS...94.3122N. doi:10.1073/pnas.94.vii.3122. PMC20332. PMID 9096356.
- ^ a b Hegan DC, Narayanan L, Jirik FR, Edelmann W, Liskay RM, Glazer PM (December 2006). "Differing patterns of genetic instability in mice deficient in the mismatch repair genes Pms2, Mlh1, Msh2, Msh3 and Msh6". Carcinogenesis. 27 (12): 2402–eight. doi:10.1093/carcin/bgl079. PMC2612936. PMID 16728433.
- ^ a b Tutt AN, van Oostrom CT, Ross GM, van Steeg H, Ashworth A (March 2002). "Disruption of Brca2 increases the spontaneous mutation rate in vivo: synergism with ionizing radiation". EMBO Rep. 3 (3): 255–60. doi:10.1093/embo-reports/kvf037. PMC1084010. PMID 11850397.
- ^ O'Hagan HM, Mohammad HP, Baylin SB (2008). Lee JT (ed.). "Double strand breaks can initiate gene silencing and SIRT1-dependent onset of Deoxyribonucleic acid methylation in an exogenous promoter CpG island". PLOS Genet. 4 (8): e1000155. doi:10.1371/periodical.pgen.1000155. PMC2491723. PMID 18704159.
- ^ Cuozzo C, Porcellini A, Angrisano T, Morano A, Lee B, Di Pardo A, Messina S, Iuliano R, Fusco A, Santillo MR, Muller MT, Chiariotti 50, Gottesman ME, Avvedimento EV (July 2007). "DNA harm, homology-directed repair, and Deoxyribonucleic acid methylation". PLOS Genet. 3 (7): e110. doi:x.1371/journal.pgen.0030110. PMC1913100. PMID 17616978.
- ^ Gerlinger M, Rowan AJ, Horswell South, Larkin J, Endesfelder D, Gronroos E, et al. (March 2012). "Intratumor heterogeneity and branched evolution revealed by multiregion sequencing". N. Engl. J. Med. 366 (10): 883–92. doi:10.1056/NEJMoa1113205. PMC4878653. PMID 22397650.
- ^ Slaughter DP, Southwick HW, Smejkal Westward (September 1953). "Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin". Cancer. half dozen (5): 963–8. doi:10.1002/1097-0142(195309)6:v<963::AID-CNCR2820060515>iii.0.CO;two-Q. PMID 13094644.
- ^ Bernstein C, Bernstein H, Payne CM, Dvorak K, Garewal H (February 2008). "Field defects in progression to gastrointestinal tract cancers". Cancer Lett. 260 (i–two): 1–ten. doi:10.1016/j.canlet.2007.11.027. PMC2744582. PMID 18164807.
- ^ Nguyen H, Loustaunau C, Facista A, Ramsey L, Hassounah N, Taylor H, Krouse R, Payne CM, Tsikitis VL, Goldschmid South, Banerjee B, Perini RF, Bernstein C (2010). "Deficient Pms2, ERCC1, Ku86, CcOI in field defects during progression to colon cancer". J Vis Exp (41): 1931. doi:10.3791/1931. PMC3149991. PMID 20689513.
- ^ Rubin H (March 2011). "Fields and field cancerization: the preneoplastic origins of cancer: asymptomatic hyperplastic fields are precursors of neoplasia, and their progression to tumors can be tracked past saturation density in civilisation". BioEssays. 33 (3): 224–31. doi:10.1002/bies.201000067. PMID 21254148. S2CID 44981539.
- ^ Tsao JL, Yatabe Y, Salovaara R, Järvinen HJ, Mecklin JP, Aaltonen LA, Tavaré Southward, Shibata D (Feb 2000). "Genetic reconstruction of private colorectal tumor histories". Proc. Natl. Acad. Sci. U.South.A. 97 (iii): 1236–41. Bibcode:2000PNAS...97.1236T. doi:x.1073/pnas.97.3.1236. PMC15581. PMID 10655514.
- ^ a b c Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW (March 2013). "Cancer genome landscapes". Science. 339 (6127): 1546–58. Bibcode:2013Sci...339.1546V. doi:ten.1126/scientific discipline.1235122. PMC3749880. PMID 23539594.
- ^ Lochhead P, Chan AT, Nishihara R, Fuchs CS, Brook AH, Giovannucci E, Ogino Due south (2014). "Etiologic field consequence: reappraisal of the field effect concept in cancer predisposition and progression". Modernistic Pathol. 28 (1): xiv–29. doi:10.1038/modpathol.2014.81. PMC4265316. PMID 24925058.
- ^ Svrcek M, Buhard O, Colas C, Coulet F, Dumont S, Massaoudi I, et al. (November 2010). "Methylation tolerance due to an O6-methylguanine Dna methyltransferase (MGMT) field defect in the colonic mucosa: an initiating step in the evolution of mismatch repair-scarce colorectal cancers". Gut. 59 (eleven): 1516–26. doi:10.1136/gut.2009.194787. PMID 20947886. S2CID 206950452.
- ^ Paluszczak J, Misiak P, Wierzbicka Chiliad, Woźniak A, Baer-Dubowska W (February 2011). "Frequent hypermethylation of DAPK, RARbeta, MGMT, RASSF1A and FHIT in laryngeal squamous cell carcinomas and adjacent normal mucosa". Oral Oncol. 47 (two): 104–vii. doi:10.1016/j.oraloncology.2010.11.006. PMID 21147548.
- ^ Zuo C, Zhang H, Spencer HJ, Vural Due east, Suen JY, Schichman SA, Smoller BR, Kokoska MS, Fan CY (October 2009). "Increased microsatellite instability and epigenetic inactivation of the hMLH1 cistron in head and cervix squamous cell carcinoma". Otolaryngol Head Neck Surg. 141 (four): 484–90. doi:ten.1016/j.otohns.2009.07.007. PMID 19786217. S2CID 8357370.
- ^ Tawfik HM, El-Maqsoud NM, Hak BH, El-Sherbiny YM (2011). "Head and neck squamous cell carcinoma: mismatch repair immunohistochemistry and promoter hypermethylation of hMLH1 gene". Am J Otolaryngol. 32 (half dozen): 528–36. doi:10.1016/j.amjoto.2010.xi.005. PMID 21353335.
- ^ Zou XP, Zhang B, Zhang XQ, Chen M, Cao J, Liu WJ (November 2009). "Promoter hypermethylation of multiple genes in early on gastric adenocarcinoma and precancerous lesions". Hum. Pathol. 40 (11): 1534–42. doi:10.1016/j.humpath.2009.01.029. PMID 19695681.
- ^ Wani Thousand, Afroze D, Makhdoomi M, Hamid I, Wani B, Bhat 1000, Wani R, Wani K (2012). "Promoter methylation status of DNA repair gene (hMLH1) in gastric carcinoma patients of the Kashmir valley". Asian Pac. J. Cancer Prev. 13 (viii): 4177–81. doi:10.7314/APJCP.2012.xiii.8.4177. PMID 23098428.
- ^ Agarwal A, Polineni R, Hussein Z, Vigoda I, Bhagat TD, Bhattacharyya S, Maitra A, Verma A (2012). "Role of epigenetic alterations in the pathogenesis of Barrett'southward esophagus and esophageal adenocarcinoma". Int J Clin Exp Pathol. 5 (five): 382–96. PMC3396065. PMID 22808291.
- ^ Hofstad B, Vatn MH, Andersen SN, Huitfeldt HS, Rognum T, Larsen S, Osnes One thousand (September 1996). "Growth of colorectal polyps: redetection and evaluation of unresected polyps for a flow of three years". Gut. 39 (3): 449–56. doi:x.1136/gut.39.3.449. PMC1383355. PMID 8949653.
- ^ Schmitt MW, Prindle MJ, Loeb LA (September 2012). "Implications of genetic heterogeneity in cancer". Ann. N. Y. Acad. Sci. 1267 (1): 110–six. Bibcode:2012NYASA1267..110S. doi:10.1111/j.1749-6632.2012.06590.ten. PMC3674777. PMID 22954224.
- ^ Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar Yard, Doyle M, FitzHugh Westward, et al. (February 2001). "Initial sequencing and analysis of the man genome" (PDF). Nature. 409 (6822): 860–921. doi:10.1038/35057062. PMID 11237011.
- ^ Yost SE, Smith EN, Schwab RB, Bao L, Jung H, Wang X, Voest E, Pierce JP, Messer M, Parker BA, Harismendy O, Frazer KA (August 2012). "Identification of high-conviction somatic mutations in whole genome sequence of formalin-stock-still breast cancer specimens". Nucleic Acids Res. 40 (14): e107. doi:10.1093/nar/gks299. PMC3413110. PMID 22492626.
- ^ Berger MF, Hodis Due east, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, et al. (May 2012). "Melanoma genome sequencing reveals frequent PREX2 mutations". Nature. 485 (7399): 502–vi. Bibcode:2012Natur.485..502B. doi:10.1038/nature11071. PMC3367798. PMID 22622578.
- ^ Roach JC, Glusman G, Smit AF, Huff CD, Hubley R, Shannon PT, et al. (Apr 2010). "Analysis of genetic inheritance in a family quartet by whole-genome sequencing". Science. 328 (5978): 636–nine. Bibcode:2010Sci...328..636R. doi:10.1126/science.1186802. PMC3037280. PMID 20220176.
- ^ Campbell CD, Chong JX, Malig M, Ko A, Dumont BL, Han L, et al. (Nov 2012). "Estimating the human mutation rate using autozygosity in a founder population". Nat. Genet. 44 (eleven): 1277–81. doi:10.1038/ng.2418. PMC3483378. PMID 23001126.
- ^ Tumor in Medical Encyclopedia
- ^ "Swelling". MedlinePlus Medical Encyclopedia. October 14, 2012.
- ^ "tumescence". Oxford English Dictionary (Online ed.). Oxford Academy Press. (Subscription or participating institution membership required.)
External links [edit]
Source: https://en.wikipedia.org/wiki/Neoplasm
0 Response to "To Go Directly to the Table of Neoplasms"
Postar um comentário